ILDs can be progressive and unpredictable1

Acute exacerbations can be fatal and strike at any time in pulmonary fibrosis1

Progressive pulmonary fibrosis is associated with decreased quality of life2
Early and regular monitoring are important to detecting progression and can help inform management decisions and patient counselling3,4

According to an online survey, physicians estimated that patients have a survival time of
2.5–4 years after detection of progressive fibrosis4
In IPF, lung function declines regardless of the clinical course of disease progression5
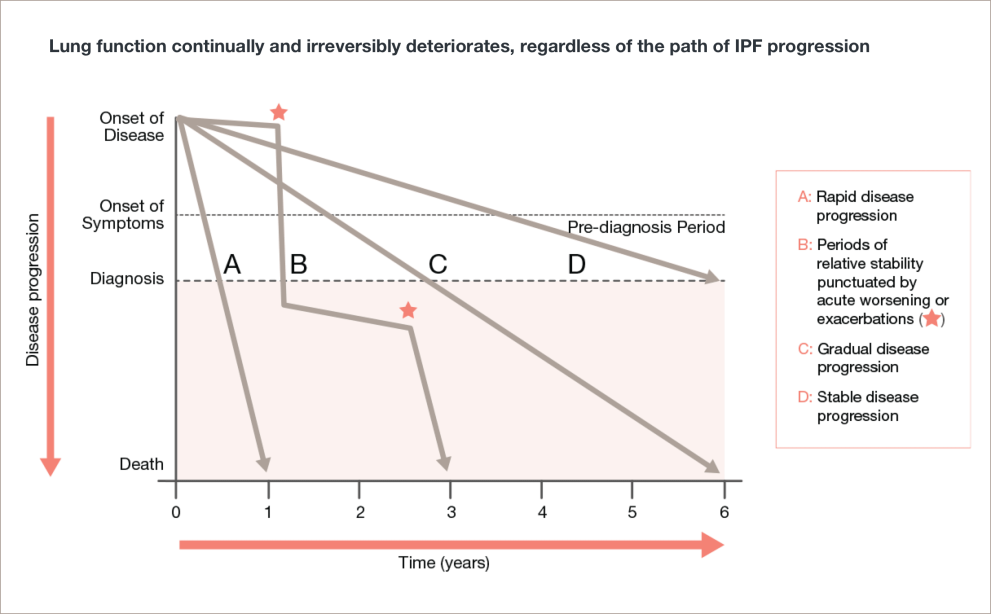
Adapted from Ley, et al.
Lung function continually and irreversibly deteriorates, regardless of the path of IPF progression5
A line graph with disease progression on the y-axis with the labels onset of disease, onset of symptoms/pre-diagnosis period, diagnosis, and death. The x-axis shows time in years from 0 to 6 years.
Line A shows rapid disease progression. The line is straight, starting at onset of disease and ends at year 1.
Line B shows periods of relative stability punctuated by acute worsening or exacerbations. The line zigzags back and forth, starting at onset of disease and ends at year 3.
Line C shows gradual disease progression. The line is straight, starting at onset of disease and ends at year 6.
Line D shows stable disease progression. The line is straight, starting at onset of disease and ends at diagnosis. It does not reach death.
In IPF, baseline FVC doesn’t predict disease progression6,7

FVC has already declined before diagnosis7

Predicted FVC can appear to be greater than 100% at baseline7

Emphysema artificially increases FVC6,7

Risk factors for progression in HP9
-
Older age
-
Smoking history
-
Lower baseline FVC % predicted
-
Lack of identification of an inciting antigen

Risk factors for progression in iNSIP10
-
Male sex
-
Initial TLC
-
Decline in FVC at 3 months
-
Presence of honeycombing on HRCT

Risk factors for progression in RA-ILD11–13
-
Extent of ILAs on HRCT
-
UIP pattern on HRCT
-
Lower baseline FVC or DLco
-
High baseline concentration
of KL-6

Risk factors for progression in SSc-ILD14–18
-
Male sex
-
Older age
-
dcSSc subtype
-
High baseline mRSS
-
Elevated KL-6 level
What should you do for patients with connective tissue disease ILD?19–22
Make regular PFTs routine in patients susceptible to connective tissue disease ILD
- Conduct proactive and regular monitoring for any deterioration in respiratory symptoms such as cough or difficulty breathing
- For at-risk patients, HRCT should be evaluated at the first suspicion of ILD involvement, if possible at baseline diagnosis, and repeated upon worsening of either PFT or respiratory symptoms
Regardless of ILD diagnosis, older patients with a UIP pattern of pulmonary fibrosis on HRCT decline at the greatest rate23
Early and regular monitoring can help detect a progressive fibrosing disease course21
Monitoring for ILD progression

PFTs6,23–25
-
Deterioration in PFTs should trigger urgency for further investigation
-
DLco is among the first parameters to decline in progressive ILDs
-
DLco decline is especially indicative of progression when accompanied by a decline in FVC or increased radiological evidence of fibrosis on HRCT over time

HRCT26–29
-
Evaluate HRCT upon worsening of PFT scores or respiratory symptoms
Other indicators for progressive fibrosis23
-
Acute exacerbation
-
Decline in the 6MWD
Time is of the essence when progressive fibrosing ILD is suspected
Refer patients with a progressive fibrosing ILD for pulmonary rehabilitation as soon as possible to help keep them active and preserve their quality of life.5
ILD=interstitial lung disease; IPF=idiopathic pulmonary fibrosis; FVC=forced vital capacity; QoL=quality of life; HP=hypersensitivity pneumonitis; iNSIP=idiopathic nonspecific interstitial pneumonia; TLC=total lung capacity; HRCT=high-resolution computed tomography; CTD-ILD=connective tissue disease-associated interstitial lung disease; RA-ILD=rheumatoid arthritis-associated interstitial lung disease; ILA=interstitial lung abnormality; DLco=diffusing capacity of the lungs for carbon monoxide; dcSSc=diffuse cutaneous systemic sclerosis; mRSS=modified Rodnan Skin Score; UIP=usual interstitial pneumonia; PFT=pulmonary function test; 6MWD=6-minute walking distance.
-
*
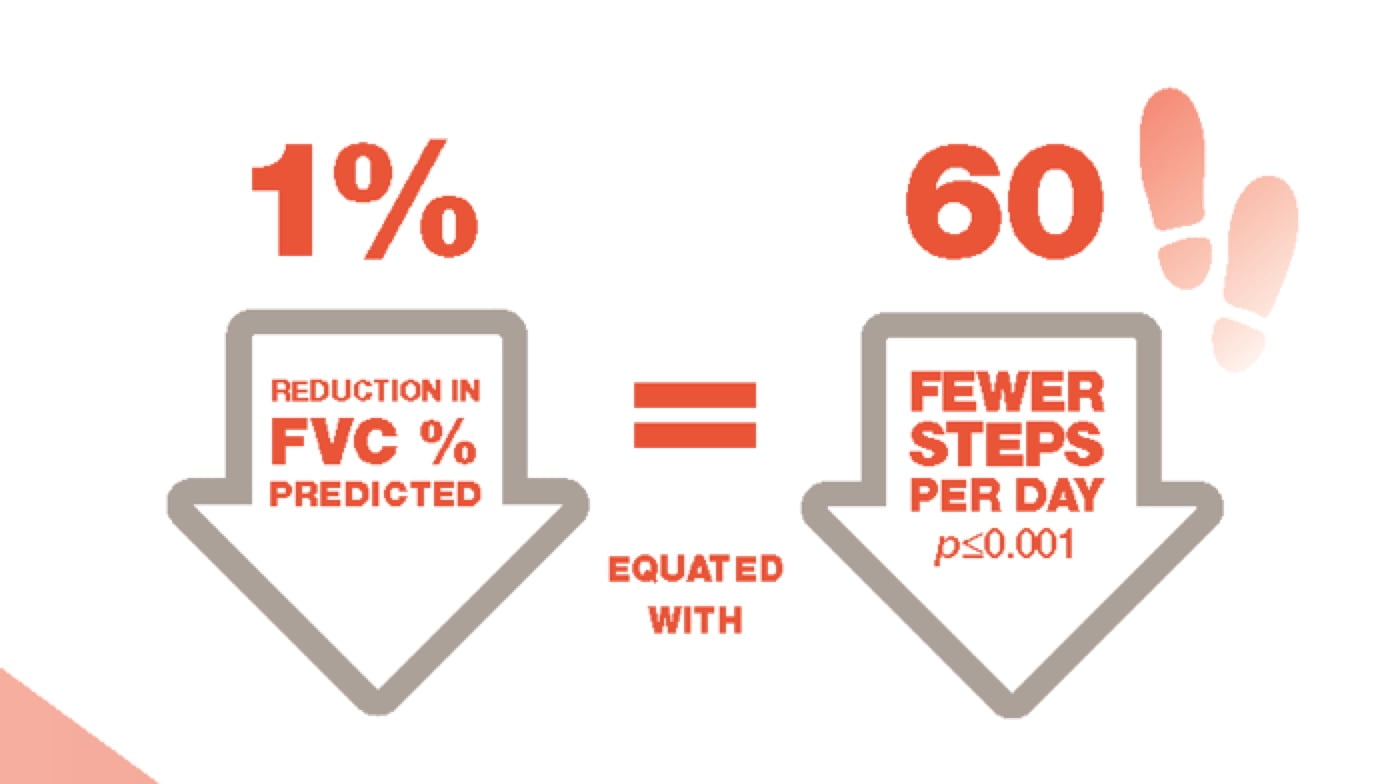
Study of 48 patients with IPF. Average steps per day: 5,017±3,360. Steps per day measured for one week, 24 hours per day, by use of the SenseWear Armband (Body, 499AMedia Inc., Pittsburgh PA, USA), an activity monitor that has been widely used in patients with IPF and chronic obstructive pulmonary disease.8
-
Kolb M, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:1–8.
-
Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. N Engl J Med 2020;383(10):958–68.
-
Cottin V, Brown KK. Interstitial lung disease associated with systemic sclerosis (SSc-ILD). Respiratory Research 2019;20(13):1–10.
-
Wijsenbeek M, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin 2019;35(11):2015–24.
-
Ley B, et al. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2011;183:431–40.
-
Raghu G, et al.; on behalf of the ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183(6):788-824.
-
Maher TM, Wuyts W. Management of fibrosing interstitial lung diseases. Adv Ther 2019;36:1518–31.
-
Bahmer T, et al. Clinical Correlates of Reduced Physical Activity in Idiopathic Pulmonary Fibrosis. Respiration 2016;91:497–502.
-
Fernández-Pérez ER, et al. Identifying an Inciting Antigen Is Associated With Improved Survival in Patients With Chronic Hypersensitivity Pneumonitis. Chest 2013;144(5):1644–51.
-
Park IN, et al. Clinical course and lung function change of idiopathic nonspecific interstitial pneumonia. Eur Respir J 2009;33:68–76.
-
Kawano-Dourado L, et al. Baseline Characteristics and Progression of a Spectrum of Interstitial Lung Abnormalities and Disease in Rheumatoid Arthritis. Chest 2020; doi: https://doi.org/10.1016/j.chest.2020.04.061.
-
Zamora-Legoff JA, et al. Progressive Decline of Lung Function in Rheumatoid Arthritis Associated Interstitial Lung Disease. Arthritis Rheumatol 2017;69(3):542–9.
-
Avouac J, et al. Improving risk-stratification of rheumatoid arthritis patients for interstitial lung disease. PLoS ONE 2020;15(5):e0232978.
-
Winstone TA, et al. Predictors of Mortality and Progression in Scleroderma-Associated Interstitial Lung Disease. Chest 2014;146(2):422–36.
-
Cappelli S, et al. Interstitial lung disease in systemic sclerosis: where do we stand? Eur Respir Rev 2015;24:411–9.
-
Nihtyanova SI, et al. Prediction of Pulmonary Complications and Long-Term Survival in Systemic Sclerosis. Arthritis & Rheumatology 2014;66(6):1625–35.
-
Wu W, et al. Prediction of progression of interstitial lung disease in patients with systemic sclerosis: the SPAR model. Ann Rheum Dis 2018;0:1–7.
-
Volkmann ER, et al. Progression of Interstitial Lung Disease in Systemic Sclerosis: The Importance of Pneumoproteins Krebs von den Lungen 6 and CCL18. Arthritis & Rheumatology 2019;71(12):2059–67.
-
Koo S-M, Uh S-T. Treatment of connective tissue disease-associated interstitial lung disease: the pulmonologist’s point of view. Korean J Intern Med 2017;32(4):600–10.
-
Wallace B, et al. Management of connective tissue diseases associated interstitial lung disease: a review of the published literature. Curr Opin Rheumatol 2016;28(3):236–45.
-
Raghu G, et al.; on behalf of the ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183(6):788–824.
-
Raghu G, et al. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer 2004;91(suppl 2):S3–S10.
-
George PM, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med 2020;8:925–34.
-
Martinez FJ, Flaherty K. Pulmonary Function Testing in Idiopathic Interstitial Pneumonias. Proc Am Thorac Soc 2006;3:315–21.
-
Le Gouellec N, et al. Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease. PLoS One 2017;12(8):e0181692.
-
Cottin V, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180076.
-
Roofeh D, et al. Management of systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol 2019;31:1–9.
-
Walsh SLF, et al. Role of imaging in progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180073.
-
Elicker BM, et al. The role of high-resolution computed tomography in the follow-up of diffuse lung disease. Eur Respir Rev 2017;26:170008.